Michael R. Hunsaker, Ph.D.
Center for Integrative Neuroscience Human Behavior; University of Utah; Salt Lake City, UT, USA
Abstract
Rodent models of spatial processing have long been used as a model for human memory deficits, particularly as related to hippocampal function. What has been long absent in these analyses, however, is a thorough description and rigorous study of the distributed neural networks associated with spatial processing-both in the human and rodent. There is a trend emerging in research to expand beyond the hippocampus for evaluating spatial memory, but the thrust of the research still focuses on the role of the hippocampus as essential and other neural substrates as performing subservient roles to the hippocampal processing. This review will describe spatial memory in terms of an attribute specificity model and demonstrate the nature of spatial processing in the rodent brain as well as describe a testable theoretical model concerning the fundamental mechanisms whereby rodents perform spatial memory tasks. In particular, a pivotal role for the retrosplenial cortex in spatial memory processing is outlined in the context of interacting memory systems.
Introduction
Since Scoville and Milner [1] described a series of patients with amygdala/hippocampal resections, the medial temporal lobe, and particularly the hippocampus, has been an area of intense interest for the study of memory in humans as well as animal models. In the late 1970s, Dave Olton and Robert Samuelson [2] developed a radial arm maze to study short term working memory and intermediate/long term reference memory in rats. In the early 1980s, Richard Morris provided a simple, easily replicable behavioral method to evaluate general memory in rats using a spatial memory task [3,4,5]. The development of this task allowed researchers an opportunity to evaluate the role of the hippocampus for spatial learning, and thus directly test the hippocampus as a cognitive map hypothesis proposed by O’Keefe and Nadel [6] concerning the role of place specific firing observed in the hippocampus of freely moving rats [7] for associative memory function and spatial cognitive mapping (cf., [8]).
Lesions to the rat hippocampus have been developed as an explicit model of the amnesia reported in patient H.M. The development of such models in rats was important as the development of similar models in non-human primates has proven difficult [9,10]. Since that time, there has been an explosion in the study of spatial memory in rat and mouse models--particularly as related to human memory dysfunction. To better design and interpret behavioral paradigms evaluating memory processing, a number of theoretical models have been proposed that classify memory into different domains. This review will describe these models and focus on a highly parallel attribute specificity model as the most flexible for the interpretation of human and rodent research. This model will then be used to describe how the brain processes spatial information and form spatial memory--the most common type of memory evaluated in rodents.
Theoretical Models of Brain Function
In order to understand the role of the hippocampus for these learning and memory processes, a number of competing theoretical models have been proposed. Around the same time the water maze was developed, Larry Squire described a dichotomous model for memory function [11,12], declarative memory dependent upon the medial temporal lobe and hippocampus, and nondeclarative memory associated with extra-medial temporal lobe and extra-hippocampal brain substrates. Declarative memory, as the name implies, referred to memories whose source can be consciously recalled and declared, or reported to the experimenter. Nondeclarative memories refer to skills, habits, priming, simple classical conditioning and non-associative learning. The binding feature of these types of memory are that there is not a source or moment in which the information was learned that can be consciously recalled. Similar models have been proposed that have similar dichotomies, albeit with different verbiage associated with the process of parsing function: hippocampus dependent explicit memory and nonhippocampus dependent implicit memory [13]; or else a hippocampal dependent declarative memory based on the representation of relationships among stimuli versus a non-hippocampal dependent procedural memory based on the representation of a single stimulus or non-relational inflexible (configural or conjunctive) configuration of stimuli, irrespective to conscious or subconscious processing [14,15].
Olton [16,17] has suggested a different dual memory system in which spatial memory can be divided into a hippocampal dependent short term working memory defined as memory for the specific personal spatial and temporal context of a situation and a non-hippocampal dependent intermediate or long term reference memory, defined as memory for rules and procedures (general knowledge) of specific situations. Different terms have been used to reflect a similar distinction in humans; particularly episodic versus semantic memory [18]. Importantly, the working memory as defined by Olton is more akin to episodic memory or short term memory rather than the working memory as commonly studied in cognitive neuroscience dependent upon the frontal cortex.
However, memory is too complex and involves many neural systems in addition to the hippocampus to be simply parsed into a dichotomous system. To remedy this situation, Kesner [19,20,21] proposed a tripartite attribute based theoretical model of memory which is organized into event-based (data-based), knowledge-based (expectation-based), and rule-based memory systems. Each system is composed of the same set of attributes or domains of memory, characterized by a set of process oriented operating characteristics and mapped onto interconnected neural circuits.
In the attribute memory model [22,23], it is proposed that the hippocampus in animals codes only spatial and temporal attributes. In humans, the right hippocampus mediates spatial and temporal attribute representations, and the left hippocampus mediates verbal and temporal attribute representations. Other models (e.g., working, explicit, and declarative memory models) propose that the hippocampus codes all information, including spatial, temporal, nonspatial (sensory cues), and egocentric spatial attributes [11-17]. Thus, models of hippocampal function vary greatly on the nature of information or attributes that are processed, coded, or represented in the hippocampus.
One view is that the hippocampus is the storage site for spatial information [6]. A different approach was taken by the working, declarative, and attribute models of memory. The working/reference memory model [16,17] posited that within every learning task there are two types of memory that organize all the critical information. It is suggested that the specific, personal, and temporal context of a situation is coded in working memory (cf., [24]). This translates into memory for events that occur on a specific trial in a task, biasing mnemonic coding toward the processing of incoming data. In contrast, information concerning rules and procedures (general knowledge) of situations is coded in reference memory. This translates into memory for events that happen on all trials in a task, biasing mnemonic coding toward the processing of expectancies based on the organization of permanently stored memory representations. The working memory model also states that hippocampus mediates working, but not reference, memory.
The attribute memory model suggests that the hippocampus is directly involved in coding of all new spatial-temporal incoming information that is likely to be relevant in trial-unique situations but that could also be of importance for all trials within a learning task (event-based memory). However, it is not involved in coding information based on expected non-varying information in the form of maps, rules, strategies, and procedures (knowledge-based memory; [23]).
It is assumed by each of these models that in new learning situations the hippocampus is involved in consolidating new information, and in familiar situations, requiring attention or memory for trial unique stimuli, and is involved in temporarily maintaining information across time. It is likely that in both situations the hippocampus maintains this information temporarily in an intermediate memory storage system.
The Attribute Model
Memory Systems for Spatial memory
At the level of processing (cf., Table 1), the event-based memory system provides for temporary representations of incoming data concerning the present (i.e., online processing), with an emphasis upon data and events that are usually personal and that occur within specific external and internal contexts. The emphasis is upon the processing of new and current information. During initial learning, emphasis is placed on the event-based memory system, which will continue to be of importance even after initial learning in situations where unique or novel trial information needs to be remembered [26,27]. This system is akin to episodic memory [12,18,27] as formulated to describe research using human subjects.
| Phase | Event-Based | Knowledge-Based | Rule-Based |
|---|---|---|---|
| Encoding | Pattern Separation | Selective Attention | Strategy Selection |
| Transient Representations | Permanent Memory Representations | ||
| Short Term Memory | Perceptual Memory | Rule Maintenance | |
| Intermediate Term Memory | |||
| Retrieval | Consolidation | Long Term Memory Rule Maintenance | |
| Pattern Completion | Retrieval Based on Flexibility and Action | Short Term Working Memory |
Table 1: Description of the processes performed by different memory systems used in the attribute theory as applicable to research using rodents
The knowledge-based memory system provides for more permanent representations of previously stored information in long-term memory and can be thought of as one’s general knowledge of the world. The knowledge-based memory system would tend to be of greater importance after a task has been learned, or given that the situation has become invariant and/or familiar. This system is akin to semantic memory [18].
The rule-based memory system receives information from the event-based and knowledge-based systems and integrates the information by applying rules and strategies for subsequent action [28,29,30,31]. In most situations, however, one would expect a contribution of all three systems with a varying proportion of involvement of one relative to the other depending primarily upon the demands of the task being performed. It is important to note that this is different than learning stimulus-response or action-outcome learning that underlies habit formation.
Specific Attributes
The attribute model makes the basic assumption that the three memory systems are composed of the same forms, domains, or attributes of memory. Even though there could be many attributes, the most important attributes int he attribute model are space, time, response, sensory-perception, and reward value (affect). In humans a language attribute is also added.
A spatial (space) attribute within this framework involves memory representations of places or relationships between places. It is exemplified by the ability to encode and remember spatial maps and to localize stimuli in external space. Memory representations of the spatial attribute can be further subdivided into specific spatial features including allocentric spatial distance, egocentric spatial distance, allocentric direction, egocentric direction, and spatial location.
A temporal (time) attribute within this framework involves memory representations of the duration of a stimulus and the succession or temporal order of temporally separated events or stimuli, and from a time perspective, the memory representation of the past.
A sensory/perceptual attribute within this framework involves memory representations of a set of sensory stimuli that are organized in the form of cues as part of a specific experience. Each sensory modality (olfaction, auditory, vision, somatosensory, and taste) can be considered part of the sensory/perceptual attribute component of memory.
A response attribute within this framework involves memory representations based on feedback from motor responses (often based on proprioceptive and vestibular cues) that occur in specific situations as well as memory representations of stimulus-response associations.
A reward value (affect) attribute within this framework involves memory representations of reward value, positive or negative emotional experiences, and the associations between stimuli and rewards.
Processes Associated with Each Attribute
Within each system attribute, information is processed in different ways based on different operational characteristics.
For the event-based memory system, specific processes involve (1) selective filtering or attenuation of interference of temporary memory representations of new information and is labeled pattern separation, (2) encoding of new information, (3) short-term and intermediate-term memory for new information, (4) the establishment of arbitrary associations, (5) consolidation or elaborative rehearsal of new information, and (6) retrieval of new information based on flexibility, action, and pattern completion.
For the knowledge-based memory system, specific processes include (1) encoding of repeated information, (2) selective attention and selective filtering associated with permanent memory representations of familiar information, (3) perceptual memory, (4) consolidation and long-term memory storage partly based on arbitrary and/or pattern associations, and (5) retrieval of familiar information based on flexibility and action.
For the rule-based memory system, it is assumed that information is processed through the integration of information from the event-based and knowledge-based memory systems for the use of major processes that include (1) the selection of strategies and rules for maintaining or manipulating information for subsequent decision making and action, (2) short-term or working memory for new and familiar information, (3) development of goals, (4) prospective coding, (5) affecting decision processes, and (6) comparing actions with expected outcomes.
Attributes Map onto Neural Substrates
On a neurobiological level (cf., Table 2; Figure 1) each attribute maps onto a set of neural regions and their interconnected neural circuits. For example, within the event-based memory system, it has been demonstrated that in animals and humans the hippocampus supports memory for spatial, temporal and language attribute information; the entire basal ganglia, particularly the dorsolateral striatum (in rodents) mediates memory for response attribute information; the amygdala and nucleus accumbens subserves memory for reward value (affect) attribute information; and the perirhinal, extrastriate visual cortex, and all other sensory cortices support memory for visual object sensory/perceptual attribute.
| Attribute | Event-Based | Knowledge-Based | Rule-Based |
|---|---|---|---|
| Spatial | Hippocampus | Parietal Cortex | Infralimbic/Prelimbic, Retrosplenial Cortex |
| Temporal | Hippocampus, Basal Ganglia | Anterior Cingulate, Infralimbic/Prelimbic | Anterior Cingulate, Infralimbic/Prelimbic |
| Sensory/Perceptual | Sensory Cortices | TE2 Cortex, Perirhinal Cortex, Piriform Cortex | Infralimbic/Prelimbic |
| Response | Caudoputamen | Precentral Cortex, Cerebellum | Precentral Cortex, Cerebellum |
| Affect | Amygdala | Agranular Insula | Agranular Insula, Infralimbic/Prelimbic |
| Executive Function | Basal Ganglia | Infralimbic/Prelimbic, Parietal Cortex | Infralimbic/Prelimbic, parietal Cortex |
| Social | Unknown Network | ||
| ProtoLanguage | Unknown Network |
Table 2: Primary neuroanatomical correlates underlying each attribute in rodents
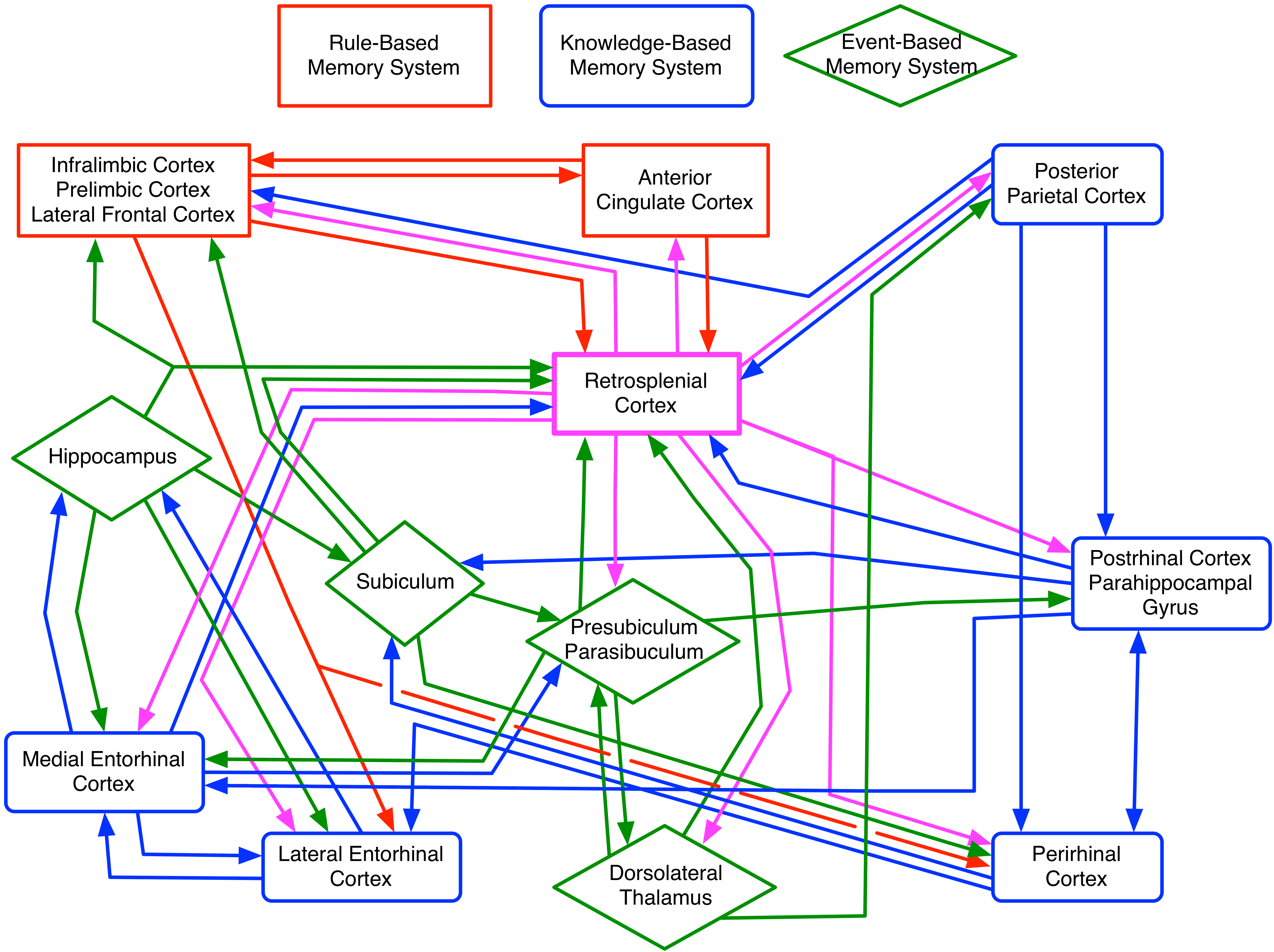
Figure 1. Neural networks underlying spatial attribute processing organized by memory system. In this model the event-based memory system (green diamonds) process incoming sensory/perceptual information to calculate the spatial attribute in an online manner. This can be simplified as an encoding process involving spatial pattern separation and rapid trial unique arbitrary associations. The knowledge based memory system (blue rounded rectangles) uses already processed spatial information from the event-based memory system to further process space. This can be simplified to a retrieval process involving spatial pattern completion, consolidation, and multiple trial learned paired patterned associations, as well as retrospective coding. The rule based memory system (red rectangles) provides the event and knowledge based memory systems with goals and reward contingencies to guide online spatial processing and retrieval processes. This can be simplified as a prospective coding process. The retrosplenial cortex (highlighted as a pink rectangle), appears based on anatomical connectivity to provide an interface for all three neural systems (cf., [112-114]).
Within the knowledge-based memory system, it has been demonstrated that in animals and humans the posterior parietal cortex supports memory for spatial attributes; the dorsal and dorsolateral prefrontal cortex and/or anterior cingulate support memory for temporal attributes; the premotor, supplementary motor, and cerebellum in monkeys and humans and precentral cortex and cerebellum in rats support memory for response attributes; the orbital prefrontal cortex and amygdala support memory for reward value (affect) attributes; the inferotemporal cortex in monkeys and humans and TE2 cortex in rats subserve memory for sensory/perceptual attributes (e.g., visual objects); and the parietal cortex, Broca and Wernicke’s areas subserve memory for the language attribute.
Within the rule-based memory system it can be shown that different subdivisions of the prefrontal cortex (and rodent homologues; [32,33,34,35]) support different attributes. For example, the dorsolateral and ventrolateral prefrontal cortex in humans support spatial, object, and language attributes and the infralimbic and prelimbic cortex in rats supports spatial and visual object attributes; the pre-motor and supplementary motor cortex in monkeys and humans and precentral cortex in rats support response attributes; the dorsal, dorsolateral, and mid-dorsolateral prefrontal cortex in monkeys and humans and anterior cingulate in rats mediate primarily temporal attributes; and the orbital prefrontal cortex in monkeys and humans and agranular insular cortex in rats support affect attributes.
Interactions Among Attributes
Despite the relative independence and parallel processing of the different attributes, it bears to mention that the neural systems that underlie memory of all forms interact and contain similar nodes (i.e., hippocampus processes space and time and in special cases can process sensory/perceptual information and affect--but all these attributes are processed in larger networks made up of disparate elements). To provide a concrete example, the interaction among temporal, spatial, and sensory/perceptual attributes will be discussed based on different interactions based on task demands.
The nature of the interactions between memory systems can be evaluated to dissect out the processes involved in both episodic and nonepisodic behavioral experiments. For illustration, two hippocampus-dependent tasks involving specific and easily identifiable sensory/perceptual stimuli (what), spatial information (where – computed from a combination of sensory/perceptual and temporal attributes), and temporal relationships between the stimuli (when) will be compared and contrasted. One task will require event-based memory processes and the other task can be solved via knowledge-based memory processes. The nature of the interactions between these three attributes corresponding to what, when, and where will be analyzed to differentiate between the two tasks.
The knowledge-based memory task requires that a pair of associations be acquired over multiple training trials. It is an object–trace–place paired-associate task involving sensory/perceptual stimuli (what), a temporal stimulus in the form of a temporal discontinuity (trace interval; when), and spatial information (where). This task is designed as follows: when a particular spatial location (a) and a Garfield toy (1) are paired across a 10 s trace interval, the animal is rewarded (a_1+). Also if a different spatial location (b) and a truck toy are matched (2), the animal is rewarded (b_2+). If spatial location (a) and the toy truck are paired, there is no reward (a_2-), similarly for spatial location (b) and (a) Garfield toy (b_1-). The trace interval separates the sensory/perceptual stimulus and the presentation of the spatial location (cf., [36]). If the association presented during a trial were rewarded, the rat would receive a reward upon displacing a block in the correct spatial location, which is then represented by the affect attribute, signaling a correct choice. This should bind the sensory/perceptual stimulus and spatial location association across the temporal discontinuity (an association involving what, when, and where). Then, the animal is presented with a new sensory/perceptual stimulus and spatial location association. If rewarded, then the process continues as before; if not rewarded, the animal does not receive any reward, and the affect attribute signals an error. Learning this task within only the event-based memory system would be difficult because the event-based memory system is susceptible to trial-by-trial interference. Both temporally adjacent (e.g., subsequent) and spatially adjacent (e.g., occurring in the same or very similar spatial locations irrespective of temporal contiguity) episodes would interfere and degrade each other during acquisition.
Learning these associations involves comparing accumulated behavioral episodes or events within the knowledge-based and rule-based memory systems to develop appropriate rules, goals, and schemas to perform the task efficiently. In other words, repeated trials must be presented if the rule and knowledge-based memory systems are to be engaged in learning and performing the task. Also, these two latter systems generate and apply abstract rules and generalize temporal, spatial, and internal contexts. In other words, the knowledge-based and rule-based systems read the accumulated behavioral episodes, clarify the relevant contextual information, and apply this information to guide future actions. Once the knowledge-based and rule-based memory systems have processed the data and generated the schemas necessary to perform the task, the event-based memory system does not significantly contribute to performance of this task since the four discriminations or associations (a_1+, b_2+, a_2-, b_1-) have been efficiently encoded and only need to be discriminated from each other [37,38].
In contrast to the above biconditional discrimination, a task developed by Morris [39] and modified by Kesner and colleagues [40] allows rats to perform a very similar sensory/perceptual stimulus and spatial location association in an event-based manner. The critical aspect of this task that requires the event-based memory system is the fact that each trial is unique, and as such there are no repeated trials across which the knowledge and rule-based memory systems can be recruited. During the study phase, the animal receives two rewarded object–place pairings (i.e., single sensory/perceptual stimulus in a spatial location defined by the sum total of sensory/ perceptual stimuli in the environment) separated by a short temporal interval. Since there are two distinct behavioral episodes in close temporal proximity to each other, information pertaining to temporal relationships between stimuli (e.g., temporal contiguity) discriminates the two episodes and facilitates retrieval [27]. During the test phase, the animal is provided with a retrieval cue. The animal has to learn that the sensory/perceptual stimulus provided as a retrieval cue is a signal to displace a neutral block in the corresponding spatial location previously paired with the cue (or to the sensory/perceptual stimulus cued by a spatial location). Since none of the 50 sensory/perceptual stimuli and 48 spatial locations are frequently paired (there are nearly 2,500 possible combinations), each pairing is trial (or behavioral episode) unique. Since the animal receives two distinct behavioral episodes followed by a retrieval cue to signal which of the two episodes needs to be recalled, the animal not only has to remember the relevant episode to receive reward, but also to discriminate between the relevant episode and the episodes presented either immediately before or after the relevant episode, as well as all previous episodes that occurred in the same or a similar spatial context.
The critical difference between the two tasks is not the cued-recall nature of the latter task per se, but that the associations to be remembered are trial unique. This allows each behavioral episode to be coded as unique, but increases potential interference from previous or subsequent behavioral episodes. To overcome this interference and to guide efficient recall of the correct behavioral episode, this task is performed with the contribution of the knowledge-based and rule-based systems such as traditional biconditional discrimination tasks, but the trial specific episodes make it necessary to depend on the event-based memory system to compare the retrieval cue to the stored episodes to efficiently recall the correct, and only the correct, behavioral episode to guide behavioral decisions and actions.
Definitions Critical to Understanding Spatial Memory
Spatial memory is a general term that is made up of a number of component processes that need to be defined prior to proceeding into how the attribute model can be used to describe these processes.
The first of these spatial features is the distinction between egocentric (or idiothetic or individual centered) and allocentric (or allothetic or world centered) reference frame. The allocentric reference frame typically refers to processing of space with reference to a constellation of landmarks or stimuli or else with respect to a standard reference angle/direction (for full mathematical description cf., [41]). A fully learned allocentric representation of an environment is what is commonly referred to as a cognitive map as defined by O’Keefe and Nadel [6]. An egocentric frame of reference can mean a number of things, but most parsimoniously it is a reference frame in which space is computed to coordinates that center on the observer or the focus of the observer’s attention. This can mean a representation linked to a single attended to beacon or landmark in space, or to observer-centered coordinates proper. A correlate to processing space using an egocentric reference frame is that the spatial representation must obligatorily be updated constantly to account for and change in the position of the observer, as once the observer has moved any remembered egocentric representation is now incorrect and no longer behaviorally relevant.
In performing calculations of space, there are four general classes of cues that can be used to generate mental representations of space that can be acted upon. The first two are the distinction between proximal and distal cues. Proximal cues refer to cues available for the subject to interact with and that can be explored from all sides. Distal cues, however, are commonly referred to as environmental cues or spatial context, and refer to cues “out there” in space that form a background or general scene within which the other types of cues are located. Importantly, it has been shown that the hippocampus and entorhinal cortex preferentially respond to changes to distal, but not proximal, cues and the parietal cortex shows the opposing pattern [42,43].
The third type of cue is actually a mental construct generated from the relationship among proximal cues. If there is a general shape made up by the proximal cues, then the parietal lobe will compute the topological relationships among the cues and the resulting general map of spatial relationships will be used to guide behavior. In other words, if the objects are in a general kite shape, then rodents will preferentially use the general shape the cues generate rather than any features of the individual cues to guide behavior [44]. This process is disrupted by parietal, but not hippocampus lesions (cf., [45]). Additionally, neuronal firing in the parietal cortex has been shown to fire specifically to topological information [46,47]. The fourth type of cue is the use of self-motion cues to generate idiothetic cues.
Also important for the understanding of spatial memory are the subtle distinction between spatial location memory, spatial distance memory, and memory for spatial context. Spatial location (or locale) memory refers to the ability to process a given specific portion of space as defined by the constellation of proximal and distal cues present in the environment. This has been shown to be performed in parallel by the hippocampus, and parietal cortex, as well as the rostral cortices (infralimbic/prelimbic among others; [28-30, 48]). Memory for spatial context refers to the ability to discriminate the constellation of proximal and distal cues present in the environment from similar cues and cue configurations in different environments. The latter is a much more computationally intensive process that has been strongly linked to hippocampus function [49,50,51].
Memory for spatial distance actually refers to a computation performed by the hippocampus at the level of the dentate gyrus--called metric processing (cf., [43-45]). This metric information is initially encoded by the dentate gyrus as a collection of egocentric distances and angles from the individual’s eye-centered field of view. This collection of egocentric distances are then combined into a spatial representation that becomes allocentric in nature. In other words, with time and hippocampal processing, what was once a collection of angles and distances between a rat and objects in space becomes independent to the rat’s location and the precise angles and distances among the items in proximal space and distal space are directly represented (cf., [43]). Similarly, grid cells in the medial entorhinal cortex have been implicated in providing the information necessary for the calculation of spatial distance.
One of the burning questions in the early days of spatial memory research was just how does an individual return to a starting point in a direct way after taking a non-direct route in space. This process is called path integration. It is the ability of an animal to integrate the distances travelled, as well as the directions taken and use that information to return to a starting point (or nest/den in the case of foraging species; [52,53,54]). At present, it is presumed that path integration in mammals makes use of the vestibular organs and other idiothetic inputs to detect and compute the animal’s movements in the three dimensions. This information is then put together with motor efferent signals, where the motor system tells the rest of the brain which movements were commanded, and optic flow, where the visual system signals how fast the visual world moves past the eyes. Information from other senses such as echolocation and magnetoception may also be integrated in animals that have access to those information. The hippocampus is the part of the brain that integrates linear and angular motion to encode a mammal's relative position in space and the medial entorhinal cortex appears to contribute to this process as well due to a robust representation of idiothetic data in the medial entorhinal neurons [55,56].
Application of the Attribute Model to Spatial Processing
The attribute model can be used to tease apart the roles for different neural networks underlying spatial processing. The role for each memory system as well as their interactions will be discussed and a model for performance on spatial memory tasks will be posited.
Event-Based Spatial Memory Processes
For processes associated with event-based memory, the focus will be on the role of circuitry associated with the hippocampus for spatial memory. With respect to specific spatial features, such as allocentric spatial distance, egocentric spatial distance, and spatial location, it has been shown in both rats and humans with bilateral hippocampal damage that there are severe deficits in event-based memory for these spatial features [57,58,59,60,61,62,63].
The spatial direction feature of spatial memory has also been investigated. Based on a delayed matching-to sample task for assessing memory for direction in rats, it was shown that hippocampal lesions disrupt memory for direction. It should be noted that medial caudate nucleus lesions also produced an impairment in memory for direction. It has been suggested in the literature suggesting that hippocampal lesions disrupt memory for the 'direction' to the goal because of impaired triangluation with the help of external landmarks, while caudate lesion disrupt memory for the direction to a beacon [64]. Additionally, it has been demonstrated that throughout the neural networks subserving event-based spatial memory (e.g., pre and post subiculum and medial entorhinal cortex among other regions) there are cell populations that show direction specific firing, called head direction cells that can be used by the rodent as an orienting mechanism (cf., [65,66,67]).
The determination of a spatial pattern separation process has been developed extensively by computational models of the subregions of the hippocampus with a special emphasis on the dentate gyrus (cf., [68,69,70]). Based on the empirical findings that all sensory inputs are processed by the dentate gyrus subregion of the hippocampus, it has been suggested that a possible role for the hippocampus might be to provide for sensory markers to demarcate spatial locations, such that the hippocampus can efficiently process spatial information (cf., [51, 70]). It is thus possible that one of the main process functions of the hippocampus is to encode and separate spatial events from each other. This would ensure that new highly processed sensory information is organized within the hippocampus and enhances the possibility of remembering and temporarily storing one place as separate from another place. It is assumed that this is accomplished via pattern separation of event information, so that spatial events can be separated from each other and spatial interference reduced. This process is akin to the idea that the hippocampus is involved in orthogonalization of sensory input information [70,71], in representational differentiation [72], and indirectly in the utilization of relationships [14].
Within the event-based memory system, the hippocampus computes overall spatial relationships within the allocentric frame by computing and combining egocentric calculations into a coherent spatial representation (perhaps via CA3 recurrent circuitry binding the egocentric and idiothetic inputs into a view invariant representation [51,70]). This is particularly important for the generations of overall space anchored to distal, rather than proximal cues.
The hippocampus-based representations involved mathematically-rich information such as precise metric relationships among stimuli (cf., Figure 2). The mathematical blueprint is generated using information from the medial entorhinal cortex and parahippocampal gyrus/postrhinal cortex. As such, this blueprint designated nodes occupied by elements, but does not specify the precise identity of the objects other than rough sketch information.
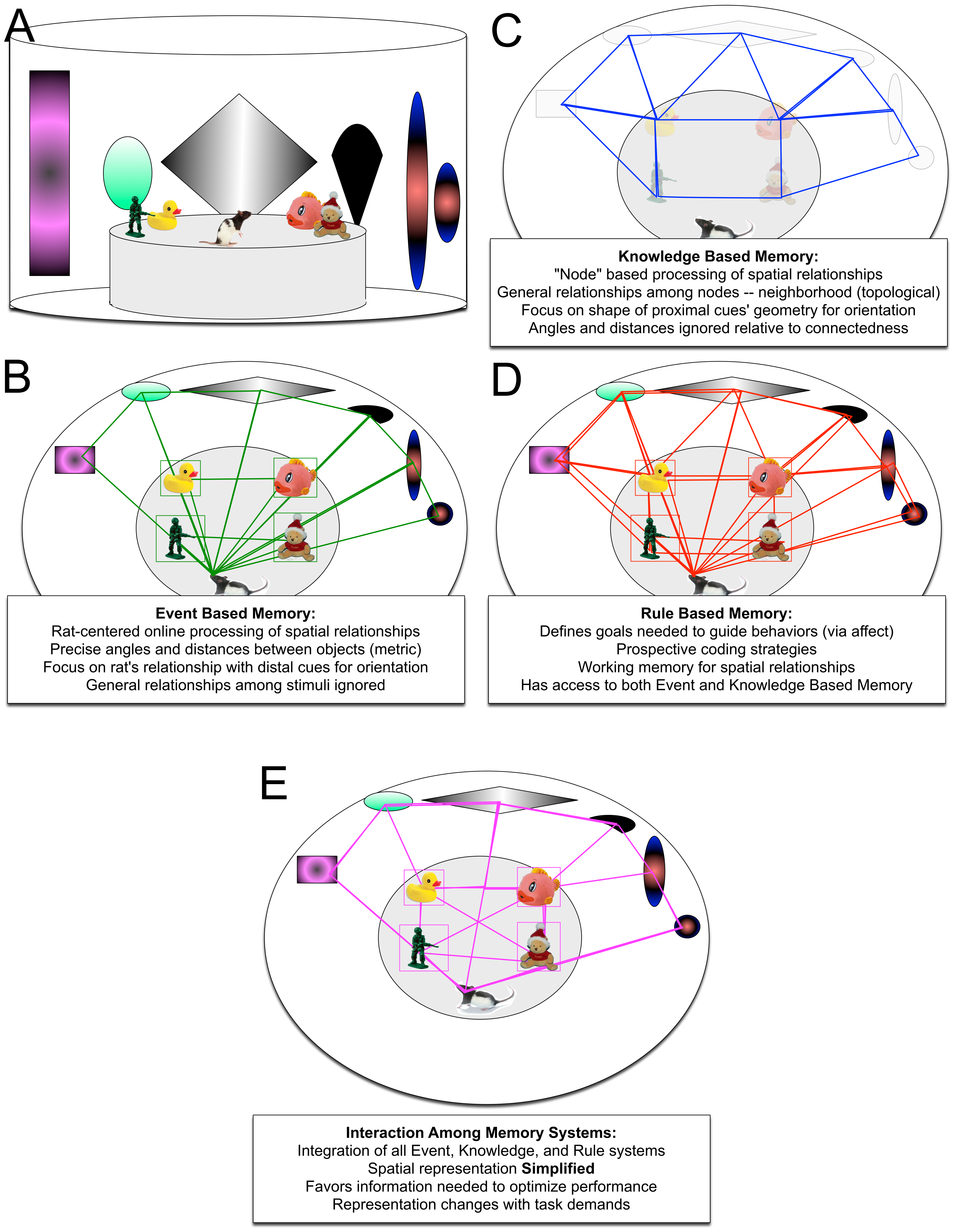
Figure 2. Model of spatial processing by interacting memory systems. A. depicts an environment in which a rat is placed to explore in a spatial memory task (cf., Goodrich-Hunsaker and colleagues, 2055, 2008). B. depicts the neural computations carried out by the event-based memory system as defined in Figure 1 with particular focus on the contributions from the hippocampus. Spatial relationships among stimuli are defined mathematically in terms of raw angles and distanced from the rat. C. depicts the neural computations undertaken by the knowledge based memory system, particular the posterior parietal cortex. Spatial relationships are defined by only the most general geometric relationships (i.e., geometric shape defined by the elements-connectedness and neighborhood), but without regard to the vantage of the rat for this processing or specific regard to which object is located at which node. D. depicts the processing by the Rule based memory system, particularly the Infralimbic/Prelimbic cortices. The rule based memory system used affective information to guide exploratory behavior and decisions undertaken by the rat. Importantly, the rule based memory system can switch between the event and knowledge based memory system as needed to guide behavioral performance. E. depicts an active interaction among the three memory systems, focusing on the proposed role for the retrosplenial cortex (cf., [112-114]). The retrosplenial cortex, having access to raw data from the thalamus, as well as the three memory systems, can perform computations in concert with the rule based memory system, as well as independently, and then signal that update to the rule based memory system. Importantly, the retrosplenial cortex simplifies the spatial inputs to those relevant to guide task performance; this process provides a mechanism whereby an animal may compute egocentric position within an allocentric frame as proposed by other authors.
Next, this mathematical or architectural blueprint generated by the hippocampus is then populated with individual objects through interactions with the rhinal cortices, particularly the perirhinal cortex and lateral entorhinal cortex. This can be viewed as a conjunctive process whereby the object and contextual information become bound into a single representation in the dentate gyrus, and as a relational process in CA3 wherein the context and object information remain separately represented to maximize flexibility in memory recall (cf., [14,40,51,73]).
Knowledge-Based Spatial Memory Processes
The posterior parietal cortex computes spatial relationships in two reference frames: a topological reference frame with reference to the local cue configurations irrespective to the distal cue configurations as well as in the egocentric frame of reference, primarily in reference to the sagittal midline of the individual, but also to single landmarks or beacons in the environment (cf., Figure 2; [74,75,76]). Counterintuitively, however, the posterior parietal cortex is also involved in spatial retrieval for allocentric information.
Rats with posterior parietal cortex lesions display deficits in both the acquisition and retention of spatial navigation tasks that are presumed to measure the operation of a spatial cognitive map within a complex environment [77,78,79]. However strong these lesion data may appear, it is worth noting that to date that there are not neurophysiological evidence for cognitive mapping in the rodent parietal lobe. They also display deficits in the acquisition and retention of spatial recognition memory for a list of five spatial locations. In a complex discrimination task in which a rat has to detect the change in location of an object in a scene, rats with posterior parietal cortex lesions are profoundly impaired [80], yet on less complex tasks involving the discrimination or short-term memory for single spatial features including spatial location, allocentric and egocentric spatial distance [81] there are no impairments. When the task is more complex, involving the association of objects and places (components of a spatial cognitive map), then posterior parietal cortex plays an important role [81,82].
Comparable deficits are found within an egocentric-allocentric distance paired associate task [81], but there is no deficit for an object-object or object-odor paired associate task, suggesting that spatial features are essential in activating and involving the posterior parietal cortex. Finally, it should be noted that in rats, neurons have been found within the posterior parietal cortex that encode spatial location and head direction information and that many of these cells are sensitive to multiple cues including visual, proprioceptive, sensorimotor and vestibular cue information [83,84].
Rule-Based Spatial Memory Processes
mPFC (infralimbic/prelimbic cortex in rodents) computes the rule based memory system data to determine the rules of the task and provide that information to the parietal and temporal lobe spatial processing systems (cf., Figure 2). These processes are associated with short term working memory processes, cross modal switching, goal-oriented control, and prospective coding (cf., [85,86,87]).
The anterior cingulate cortex and agranular insula compare the behavioral outputs with expected rewards, affective contingencies, etc and signal any mismatch to the infralimbic/prelimbic cortex for the rule to be modified (cf., [88,89]).
Rats with lesions of the infralimbic/prelimbic cortex, but not of the anterior cingulate and precentral cortex, fail to acquire an object-place association [88]. In a subsequent study Lee and Solivan [90] showed that temporary inactivation of the infralimbic/prelimbic cortex led to profound impairments in an object-place paired association task. Furthermore, impairment in a novelty detection paradigm using an object-in-place learning task has been observed in in rats with infralimbic/prelimbic cortex lesions [91]. In the context of other types of paired associate learning, Petrides [92] has shown that humans with prefrontal cortex lesions have difficulty in learning a paired associate task and Pigott and Milner [93] reported that frontal lobe damaged patients are impaired for objects and places in a complex visual scene task.
The infralimbic/prelimbic cortex appears to play an important role in working memory for visual object and spatial location information. Supporting evidence is based on the findings that lesions of the infralimbic/prelimbic cortex produce deficits in working memory for spatial information [88,89], and working memory for visual object information [89, 94,95]. However, infralimbic/prelimbic cortex lesions do not produce a deficit in working memory for a food reward [96,97]. Further support of this conclusion was reported by Chang and colleagues [98], who found sustained neural firing in the infralimbic/prelimbic cortex during the delay within a delayed matching-to-position task, and Baeg and colleagues [99] who recorded from the infralibmic/prelimbic cortex in a spatial delayed alternation task reported an increase in neural firing during the delay period. Importantly, it is becoming clear that the infralimbic/prelimbic cortices influence cellular firing in the hippocampus by biasing the firing patterns to goal locations or other task relevant factors other than place per se (cf., [100]). These findings are interpreted as the infralimbic/prelimbic cortices being critical for the computation of planned trajectories and modification of behavioral output along those pre-planned goals [101,102,103,104,105].
Independence of Memory Systems
Rats with posterior parietal cortex lesions are impaired in an implicit spatial repetition priming experiment but perform without difficulty in processing positive priming for features of visual objects and a short-term or working memory for a spatial location experiment [106], suggesting that the posterior parietal cortex plays a role in spatial perceptual memory within the knowledge-based memory system, but does not play a role in spatial memory within the event-based memory system.
Additionally, Goodrich-Hunsaker and colleagues [44] demonstrated that the hippocampus, but not the posterior parietal cortex processed metric relationships among proximal stimuli. However, the posterior parietal cortex, but not the hippocampus, processed topological relationships among proximal stimuli. These findings functionally doubly dissociated the event and knowledge-based memory system spatial processes. Goodrich-Hunsaker and colleagues [107] also demonstrated that the parietal lobe mediates the processing of topological relationships among elements that make up stimuli using a task modified from one used to study topological processing deficits in Balint’s syndrome [108].
In humans there is a general loss of topographic sense, which may involve loss of long-term geographical knowledge as well as an inability to form cognitive maps of new environments. Using PET scanning and functional MRI data, it can be shown that complex spatial information results in activation of the parietal cortex [109]. Furthermore, in patients with parietal lesions and spatial neglect, there is a deficit in spatial repetition priming without a loss in short term or working memory for spatial information [110]. Additionally, Keane, and colleagues [111] reported that a patient with occipital-lobe damage (extending into parietal cortex) showed a deficit in perceptual priming but had no effect on recognition memory, whereas a patient with bilateral medial temporal lobe damage (including hippocampus) had a loss of recognition memory, but no loss of perceptual memory.
Integration Among Memory Systems
The Rule based memory system sends projections into the elements processing the event and knowledge based memory systems. (i.e., posterior parietal cortex and parahippocampal gyrus/hippocampus)
Importantly, the retrosplenial cortex is in a unique location to integrate head direction information from the thalamus and subicular complex (pre/parasubiculum), metric/distal allocentric space from the hippocampus and event-based memory system and topologic/proximal space from the parietal cortex and knowledge based memory system as well as reciprocal connectivity with the anterior cingulate, infralimbic/prelimbic cortices, agranular insula, and rodent homologue to the dorsolateral prefrontal cortex (cf., Figure 1). This allows the retrosplenial cortex to not only act upon incoming sensory information, but also to send the necessary signals to the rostral cortices to modulate/influence top down signals that guide behavior
This puts the retrosplenial cortex in a unique location to not only integrate the event and knowledge based memory systems or to compute egocentric location in allocentric space as has been proposed [112,113]; but also to actively switch among pure egocentric, pure allocentric, and integrations involving combinations of the two-catered to the demands of each particular behavioral context (cf., [114]; Figure 2).
The obvious benefit of having a structure in this location is that it allows full integration of multiple spatial reference frames in a manner that is capable of changing among many different states or forms depending upon the behavioral context [115,116,117]. Additionally, it is likely that the retrosplenial cortex, in performing this integration removes redundancy from the spatial representation, which results in a parsimonious map sufficient to drive behavioral output, but not containing the resolution of the spatial map computed within the event-based memory system or elegant geometric representation of the knowledge based memory system. Tasks requiring higher resolution metric information or demanding topological representations will result in the rule based memory system biasing the integration to favor those modalities.
This is not a trivial point because the dentate gyrus in the hippocampus will always compute an orthogonal representation of space based on a metric representations of distal cues and mathematical relationships among the local cues and the distal cues in relation to the location of the rodent, whether the behavioral situation demands such a map or not. In parallel, the parietal lobe will always compute a topological, or egocentric space with a particular focus on proximal cue configurations, even when doing so may be disruptive to performance of the behavioral or spatial task at hand. As such, neither the hippocampus nor parietal readout is sufficient to guide performance on behavioral tasks, even with the input from the rostral cortices with rule based information, as neither the posterior parietal cortex nor the hippocampus have the others’ spatial information.
Additionally, the retrosplenial cortex receives information pertaining to the sensory/perceptual and temporal attributes, as well as affective information via the infralimbic/prelimbic cortices. These information from nonspatial attributes facilitate the identification of behavioral context, time of day, or affective motivation; all information useful for the optimization of task performance (cf., [115,117]).
The retrosplenial cortex, however, having a robust connectivity with both hippocampus as well as parietal cortex, has relatively complete access to both types of information, as well as independently derived information pertaining to head direction from the thalamus and pre/parasubiculum (leading to retrosplenial neurons showing direction-based firing themselves; cf., [115]). As such, with inputs from the anterior cingulate and infralimbic/prelimbic, the retrosplenial cortex can bias the integrated map toward topologic over metric information or ego over allocentric information as needed, or vice versa as situations demand (for evidence for infralimbic/prelimbic cortices influencing hippocampus cellular activity cf., [101,105]). Additionally, the retrosplenial cortex can transmit information pertaining to the map being computed to the rostral cortices (esp. anterior cingulate, infralimbic/prelimbic, anterior insula, and rodent homologue to the dorsolateral prefrontal cortex) to inform the rule based memory system pertaining to the maps being used to guide behavior [113].
Conclusions
Spatial processing is a complex process mediated by a large network of structures located throughout the brain. Using the attribute model one can parse the networks into smaller sub-networks that can be studied in turn. The advantage of using a theoretical model such as the attribute model is that the resulting descriptions of information processing lend themselves to empirical study since the model assumes massively parallel processing across memory systems.
Using the attribute model, a role for the retrosplenial cortex for spatial processing can be proposed and tests of the theory may be designed. Specifically, following proposals of Aggleton and colleagues [112,113] as well as Petrides and colleagues [114], the retrosplenial cortex is assumed to provide a two-way interface among the event-based, knowledge-based, and rule-based memory system in such a manner as to optimize behavioral performance. Importantly, the proposed role for the retrosplenial cortex for spatial processing can be easily tested using behavioral methods. Based on the proposed role for the retrosplenial cortex as an interface between the event-based and knowledge-based memory systems, it seems logical that evaluating spatial processing using tests of event-based, knowledge-based, and tests of the event-based and knowledge-based memory systems’ interactions would serve to tease apart the role for the retrosplenial cortex more precisely than previously possible.
References:
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957; 20(1):11-21.
- Olton DS, Samuelson RJ. Remembrance of places past. J Exp Psych Animal Behav Process. 1976; 2(2):97-116.
- Morris RGM. Spatial localisation does not depend on the presence of local cues. Learning and Motivation 1981; 12:239-260.
- Morris RGM, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature 1982; 297(5868):681-683.
- Sutherland RJ, Dyck RH. Place navigation by rats in a swimming pool. Can J. Psychol 1984; 38:322-247.
- O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. 1978. Oxford University Press.
- O'Keefe J. Place units in the hippocampus of the freely moving rat. Exp. Neurol. 1976;51:78-109.
- Tolman EC. Cognitive maps in rats and men. Psychol Rev. 1948; 55(4):189-208.
- Zola-Morgan S, Squire LR. Medial temporal lesions in monkeys impair memory on a variety of tasks sensitive to human amnesia. Behav Neurosci. 1985; 99(1):22-34.
- Tabuchi E, Endo S, Ono T, Nishijo H, Kuze S, Kogure K. Hippocampal neuronal damage after transient forebrain ischemia in monkeys. Brain Res Bull. 1992; 29(5):685-90
- Squire LR. Declarative and nondeclarative memory: Multiple brain systems supporting learning and memory. In D. L. Schacter & E. Tulving (Eds.), Memory systems 1994 (pp. 203-231). Cambridge: MIT Press.
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Ann Rev Neurosci, 2004; 27:279-306.
- Schacter DL. Implicit memory: History and current status. J Exp Psychol: Learn, Mem, Cognition, 1987; 13:501-518.
- Cohen NJ, Eichenbaum HB. Memory, amnesia, and hippocampal function. 1993. Cambridge: MIT Press.
- Eichenbaum, H. Hippocampus: Cognitive processes and neural representations that underlie declarative memory. Neuron, 2004; 44:109-120.
- Olton DS. Memory functions and the hippocampus. In W. Seifert (Ed.), Neurobiology of the hippocampus. 1983. New York: Academic.
- Olton DS. Hippocampal function and memory for temporal context. In R. L. Isaacson & K. H. Pribram (Eds.), The hippocampus (Vol. 3). 1986. New York: Plenum.
- Tulving E.. Elements of episodic memory. 1983. Oxford: Clarendon.
- Kesner RP. Neurobiological views of memory. In J. L. Martinez & R. P. Kesner (Eds.), Neurobiology of learning and memory (pp. 361-416). 1998. San Diego: Academic.
- Kesner RP. Memory neurobiology. In V. S. Ramachadran (Ed.), Encyclopedia of the human brain (Vol. 2, pp. 783-796). 2002. San Diego: Academic.
- Kesner RP. Neurobiological foundations of an attribute model of memory. Comparative cognition and behavior reviews. 2013; 8:29-59.
- Kesner RP. Neurobiological views of memory. In: R.P. Kesner & J.L. Martinez (Eds.), The neurobiology of learning and memory, 2nd Ed. (pp. 271-304). 2007. San Diego, CA: Academic Press.
- Kesner RP, DiMattia BV. Neurobiology of an attribute model of memory. Progress in Psychobiology and Physiological Psychology. A. R. Morrison and A. N. Epstein eds., pp. 207-277; 1987.
- Rawlins, J. N. P. Associations across time: the hippocampus as a temporary memory store. Behav. Brain Sci. 1985; 8:479-96.
- Kesner RP, Hunsaker MR. The temporal attributes of episodic memory. Behav Brain Res. 2010; 215(2):299-309.
- Hunsaker MR. The importance of considering all attributes of memory in behavioral endophenotyping of mouse models of genetic disease. Behav Neurosci. 2012; 126(3):371-80.
- Hunsaker MR, Kesner RP. The attributes of episodic memory processing. In: E. Dere, J. P. Huston, A. Easton, and L. Nadel (Eds). Handbook of Behavioral Neuroscience, Vol. 18, Episodic memory research. 2008. Amsterdam: Elsevier, pp. 57-79.
- Kesner RP, Churchwell JC. An analysis of rat prefrontal cortex in mediating executive function. Neurobiol Learn Mem. 2011; 96(3):417-31.
- Churchwell JC, Kesner RP. Hippocampal-prefrontal dynamics in spatial working memory: interactions and independent parallel processing. Behav Brain Res. 2011; 225(2):389-95.
- Churchwell JC, Morris AM, Musso ND, Kesner RP. Prefrontal and hippocampal contributions to encoding and retrieval of spatial memory. Neurobiol Learn Mem. 2010; 93(3):415-21.
- Churchwell JC, Morris AM, Heurtelou NM, Kesner RP. Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Behav Neurosci. 2009; 123(6):1185-96.
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003; 146(1-2):3-17.
- Rose JE, Woolsey CN. The orbitofrontal cortex and its connections with the mediodorsal nucleus in rabbit, sheep and cat. Res Publ Assoc Res Nerv Ment Dis. 1948;27(1 vol.):210-32.
- Rose JE, Woolsey CN. Structure and relations of limbic cortex and anterior thalamic nuclei in rabbit and cat. J Comp Neurol. 1948; 89(3):279-347.
- Preuss TM. "Do rats have prefrontal cortex? The Rose-WoolseyAkert program reconsidered." J Cog Neurosci. 1995; 7(1):1–24.
- Hunsaker MR, Thorup JA, Welch T, Kesner RP. The role of CA3 and CA1 in the acquisition of an object-trace-place paired associate task. Behav. Neurosci. 2006; 120:1252–1256.
- O’Reilly RC, Frank MJ. Making working memory work: a computational model of learning in the prefrontal cortex and basal ganglia. Neural Comput. 2006; 18:283–328.
- O’Reilly RC, Rudy JW. Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psych. Rev. 2001; 108:311–345.
- Day M, Langston R. Morris RGM. Glutamate-receptor-mediated encoding and retrieval of paired-associate learning. Nature. 2003; 424:205–209.
- Kesner RP, Hunsaker MR, Warthen MW. The CA3 subregion of the hippocampus is critical for episodic memory processing by means of relational encoding in rats. Behav Neurosci. 2008; 122(6):1217-25.
- Redish, A.D. Beyond the Cognitive Map: From Place Cells to Episodic Memory. 1999. MIT Press, Cambridge.
- Poucet B. Object exploration, habituation, and response to a spatial change in rats. Behav. Neurosci., 1989; 103:1009–1016.
- Poucet B. Spatial cognitive maps in animals: new hypothesis on their structure and neural mechanisms. Psych. Rev., 1993; 100:163–182.
- Cheng K. A purely geometric module in the rat’s spatial representation. Cognition, 1986; 23:149–178.
- Goodrich-Hunsaker NJ, Hunsaker MR, Kesner RP. Dissociating the role of the parietal cortex and dorsal hippocampus for spatial information processing. Behav. Neurosci. 2005; 119:1307–1375.
- Nitz DA. Spaces within spaces: rat parietal cortex neurons register position across three reference frames. Nat Neurosci. 2012; 15(10):1365-7.
- Nitz DA. Tracking route progression in the posterior parietal cortex. Neuron. 2006; 49(5):747-56.
- Kesner RP. Subregional analysis of mnemonic functions of the prefrontal cortex in the rat. Psychobiology. 2000; 28:219-228.
- Rolls ET. A theory of hippocampal function in memory. Hippocampus. 1996; 6:601–620.
- Rolls ET. Functions of neuronal networks in the hippocampus and neocortex in memory. In J. H. Bryne & W.O. Berry (Eds.), Neural models of plasticity: Experimental and theoretical approaches (pp. 240–265). 1989. San Diego: Academic.
- Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog Neurobiol. 2006; 79:1-48.
- Mittelstaedt H. Triple-loop model of path control by head direction and place cells. Biol Cybern. 2000; 83(3):261-70.
- Mittelstaedt ML, Mittelstaedt H. Idiothetic navigation in humans: estimation of path length. Exp Brain Res. 2001; 139(3):318-32.
- Vickerstaff RJ, Di Paolo EA. Evolving neural models of path integration. J Exp Biol. 2005; 208(Pt 17):3349-66.
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the 'cognitive map'. Nat Rev Neurosci. 2006; 7(8):663-78.
- Long JM, Kesner RP. Effects of hippocampal and parietal cortex lesions on memory for egocentric distance and spatial location information in rats. Behav Neurosci. 1998; 112(3):480-95.
- Long JM, Kesner RP. The effects of dorsal versus ventral hippocampal, total hippocampal, and parietal cortex lesions on memory for allocentric distance in rats. Behav Neurosci. 1996; 110(5):922-32.
- Kesner RP, Hunt ME, Williams JM, Long JM. Prefrontal cortex and working memory for spatial response, spatial location, and visual object information in the rat. Cereb Cortex. 1996; 6(2):311-8.
- Goodrich-Hunsaker NJ, Hopkins RO. Spatial memory deficits in a virtual radial arm maze in amnesic participants with hippocampal damage. Behav Neurosci. 2010; 124(3):405-13.
- Kesner RP, Goodrich-Hunsaker NJ. Developing an animal model of human amnesia: the role of the hippocampus. Neuropsychologia. 2010; 48(8):2290-302.
- Goodrich-Hunsaker NJ, Livingstone SA, Skelton RW, Hopkins RO. Spatial deficits in a virtual water maze in amnesic participants with hippocampal damage. Hippocampus. 2010; 20(4):481-91.
- Goodrich-Hunsaker NJ, Gilbert PE, Hopkins RO. The role of the human hippocampus in odor-place associative memory. Chem Senses. 2009; 34(6):513-21.
- DeCoteau WE, Hoang L, Huff L, Stone A, Kesner RP. Effects of hippocampus and medial caudate nucleus lesions on memory for direction information in rats. Behav Neurosci. 2004; 118:540-545.
- Taube JS. Head direction cell firing properties and behavioural performance in 3-D space. J Physiol. 2011; 589(Pt 4):835-41.
- Muir GM, Taube JS. The neural correlates of navigation: do head direction and place cells guide spatial behavior? Behav Cogn Neurosci Rev. 2002; 1(4):297-317.
- Taube JS. The head direction signal: origins and sensory-motor integration. Annu Rev Neurosci. 2007; 30:181-207.
- Myers CE, Scharfman HE. Pattern separation in the dentate gyrus: a role for the CA3 backprojection. Hippocampus. 2011; 21(11):1190-215.
- Myers CE, Scharfman HE. A role for hilar cells in pattern separation in the dentate gyrus: a computational approach. Hippocampus. 2009; 19(4):321-37.
- Hunsaker MR, Kesner RP. The operation of pattern separation and pattern completion processes associated with different attributes or domains of memory. Neurosci Biobehav Rev. 2013; 37(1):36-58.
- Yassa MA, Stark CEL. Pattern separation in the hippocampus. Trends Neurosci. 2011; 34:515-525.
- Myers CE, Gluck MA, Granger R. Dissociation of hippocampal and entorhinal function in associative learning: A computational approach. Psychobiology. 1995; 23:116-138.
- Hunsaker MR, Mooy GG, Swift JS, Kesner RP. Dissociations of the medial and lateral perforant path projections into dorsal DG, CA3, and CA1 for spatial and nonspatial (visual object) information processing. Behav Neurosci, 2007; 121:742-750.
- Rogers JL, Kesner RP. Hippocampal-parietal cortex interactions: evidence from a disconnection study in the rat. Behav Brain Res. 2007; 179(1):19-27.
- Rogers JL, Kesner RP. Lesions of the dorsal hippocampus or parietal cortex differentially affect spatial information processing. Behav Neurosci. 2006; 120(4):852-60.
- Kesner RP, Rogers JL. An analysis of independence and interactions of brain substrates that subserve multiple attributes, memory systems, and underlying processes. Neurobiol Learn Mem. 2004; 82(3):199-215.
- DiMattia BV, Kesner RP. The role of the posterior parietal association cortex in the processing of spatial event information. Behav Neurosci. 1998; 102:397-403
- DiMattia BV, Kesner RP. Spatial cognitive maps: Differential role of parietal cortex and hippocampal formation. Behav Neurosci, 1998; 102:471-480.
- Kesner RP, Farnsworth G, Kametani H. Role of parietal cortex and hippocampus in representing spatial information. Cereb Cortex. 1992; 1:367-373.
- DeCoteau WE, Kesner RP. Effects of hippocampal and parietal cortex lesions on the processing of multiple object scenes. Behav Neurosci. 1998; 112:68-82.
- Long JM, Kesner RP. The effects of dorsal vs. ventral hippocampal, total hippocampal, and parietal cortex lesions on memory for allocentric distance in rats. Behav Neurosci. 1996; 100:922-932.
- Long JM, Kesner RP. The effects of parietal cortex lesions on an object/spatial location paired-associate task in rats. Psychobiology 1998; 26:128-133.
- Chen LL., Lin L, Barnes CA, McNaughton BL. Head-direction cells in the rat posterior cortex II: Contributions of visual and ideothetic information to the directional firing. Exp Brain Res. 1994; 101:24-34.
- McNaughton BL, Chen LL, Marcus EJ. "Dead reckoning", landmark learning, and the sense of direction: A neurophysiological and computational hypothesis. J Cog Neurosci. 1991; 3:190-202.
- Young JJ, Shapiro, ML Dynamic coding of goal-directed paths by orbital prefrontal cortex. J Neurosci. 2011; 31(16):5989-6000.
- Rich EL, Shapiro ML. Abstract rule coding by prefrontal cortical neurons in the rat. J Neurosci. 2009; 29(22):7208-7219.
- Young JJ, Shapiro ML. Double dissociation and hierarchical organization of strategy switches and reversals in the rat PFC. Behav Neurosci, 2009; 123(5):1028-35.
- Kesner RP, Ragozzino ME. The role of the prefrontal cortex in object-place learning: A test of the attribute specificity model. Behav Brain Res. 2003; 146:159-165.
- Ragozzino ME, Kesner RP. The role of rat dorsomedial prefrontal cortex in working memory for egocentric responses. Neurosci Lett. 2001; 308:145-148.
- Lee I, Solivan F. The roles of the medial prefrontal cortex and hippocampus in a spatial paired-association task. Learn Mem. 2008; 15:357-367.
- Barker GRI, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: A disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci. 2007; 27:2948-2957.
- Petrides M.. Deficits on conditional associate learning tasks after frontaland temporal-lobe lesions in man. Neuropsychologia, 1985; 23:601-614.
- Pigott S, Milner B. Memory for different aspects of complex visual scenes after unilateral temporalor frontal-lobe resection. Neuropsychologia, 1993; 31:1-15.
- Di Pietro NC, Black YD, Green-Jordan K, Eichenbaum HB, Kantak KM. Complementary tasks to measure working memory in distinct prefrontal cortex subregions in rats. Behav Neurosci. 2004; 118:1042-1051.
- Ragozzino ME, Detrick S, Kesner RP. The effects of prelimbic and infralimbic lesions on working memory for visual objects in rats. Neurobiol Learn Mem. 2001; 77:29-43.
- DeCoteau WE, Kesner RP, Williams JM. Short-term memory for food reward magnitude: The role of the prefrontal cortex. Behav Brain Res.1997; 88:239-249.
- Ragozzino ME, Kesner RP. The role of the agranular insular cortex in working memory for food reward value and allocentric space in rats. Behav Brain Res. 1999; 98:103-112.
- Chang JY, Chen L, Luo F, Shi LH, Woodward DJ. Neuronal responses in the frontal cortico-basal ganglia system during delayed matching-to-sample task: Ensemble recording in freely moving rats. Exp Brain Res. 2002; 142:67-80.
- Baeg EH, Kim YB, Huh K, Mook-Jung I, Kim HT, Jung MW. Dynamics of population code for working memory in the prefrontal cortex. Neuron. 2003; 40:177-188.
- Blanquat PD, Hok V, Save E, Poucet B, Chaillan FA. Differential role of the dorsal hippocampus, ventro-intermediate hippocampus, and medial prefrontal cortex in updating the value of a spatial goal. Hippocampus. 2013.
- Hok V, Chah E, Save E, Poucet B. Prefrontal cortex focally modulates hippocampal place cell firing patterns. J Neurosci. 2013; 33(8):3443-51.
- de Saint Blanquat P, Hok V, Alvernhe A, Save E, Poucet B. Tagging items in spatial working memory: a unit-recording study in the rat medial prefrontal cortex. Behav Brain Res. 2010; 209(2):267-73.
- Burton BG, Hok V, Save E, Poucet B. Lesion of the ventral and intermediate hippocampus abolishes anticipatory activity in the medial prefrontal cortex of the rat. Behav Brain Res. 2009; 199(2):222-34.
- Hok V, Lenck-Santini PP, Roux S, Save E, Muller RU, Poucet B. Goal-related activity in hippocampal place cells. J Neurosci. 2007; 27(3):472-82.
- Hok V, Save E, Lenck-Santini PP, Poucet B. Coding for spatial goals in the prelimbic/infralimbic area of the rat frontal cortex. Proc Natl Acad Sci U S A. 2005; 102(12):4602-7.
- Chiba AA, Kesner RP, Jackson P. Two forms of spatial memory: A double dissociation between the parietal cortex and the hippocampus in the rat. Behav Neurosci. 2002; 116:874-883.
- Goodrich-Hunsaker NJ, Howard BP, Hunsaker MR, Kesner RP. Human topological task adapted for rats: Spatial information processes of the parietal cortex. Neurobiol Learn Mem. 2008; 90(2):389-394.
- Robertson LC, Treisman A, Friedman-Hill S, Grabowecky M.The interaction of spatial and object pathways: Evidence from Balint's syndrome. J Cog Neurosci. 1997; 9:295-317.
- Ungerleider LG. Functional brain imaging studies of cortical mechanisms of memory. Science. 1995; 270:769-775.
- Ellis AX, Sala SD, Logie RH. The Bailiwick of visuo-spatial working memory: evidence from unilateral spatial neglect. Cog Brain Res. 1996; 3:71-78.
- Keane MM, Gabrieli JDE, Mapstone HC, Johnson KA, Corkin S. Double dissociation of memory capacities after bilateral occipital-lobe or medial temporal-lobe lesions. Brain. 1995; 118:1129-1148.
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009; 10(11):792-802.
- Aggleton JP. Understanding retrosplenial amnesia: insights from animal studies. Neuropsychologia. 2010; 48(8):2328-38.
- Iaria G, Chen JK, Guariglia C, Ptito A, Petrides M. Retrosplenial and hippocampal brain regions in human navigation: complementary functional contributions to the formation and use of cognitive maps. Eur J Neurosci. 2007; 25(3):890-9.
- Cooper BG, Manka TF, Mizumori SJ. Finding your way in the dark: the retrosplenial cortex contributes to spatial memory and navigation without visual cues. Behav Neurosci. 2001; 115(5):1012-28.
- Cooper BG, Mizumori SJ. Temporary inactivation of the retrosplenial cortex causes a transient reorganization of spatial coding in the hippocampus. J Neurosci. 2001; 21(11):3986-4001.
- Cooper BG, Mizumori SJ. Retrosplenial cortex inactivation selectively impairs navigation in darkness. Neuroreport. 1999; 10(3):625-30.